Returning to normality after vaccination in 2021
- González Burguete Group
.jpg/v1/fill/w_320,h_320/file.jpg)
- Jun 11, 2021
- 15 min read
Updated: Sep 21, 2023
After 17 months since the world saw the spread of a new virus called COVID-19 (SARS CoV-2), the world has been forced to place their citizens in containment for all this time. The only countries that were able to break containment were those which managed the spread by the use of mandatory mask like Taiwan or by closing their borders like Australia. However, currently countries like Israel and UK have reduce the number of deaths due to effective application of vaccinations to their citizens.
The vaccination is the only way that countries have to control the spread of the disease. I do hope that the seriousness of the pandemic and the timely use of the vaccination could convince the world of the effectiveness and the usefulness of the vaccine. However, it is important to remember that the vaccine just reduces the effects of the disease if you get it, but it not a cure as those take longer to be developed.
It is curious that it has taken a pandemic for me to be interested in how vaccines work and how they are developed. In any case as this has been the major topic of conversation since the pandemic happen, I thought that it would be valuable topic to research.
The vaccine development and testing process is divided into three stages with very distinctive goals: Stage 1 consists of the laboratory research and animal testing, Stage 2 consists of the clinical studies, and Stage 3 consists of the approval and licensure. The vaccine development is a very long and complex process which usually takes between 10 to 15 years with the current system developed during the 20th century which involves standardizing their procedures and regulations (source: historyofvaccines.org).
Stage 1 of the development is the laboratory and animal testing, which usually takes between 2 and 4 years. This is the initial stage of the research and is divided in three parts starting by studying the disease and ending with the necessary paperwork to start developing a new drug for humans.
The first part is the exploratory stage and consists in laboratory research which usually involves government and academically funded as their interest is a common one in order to identify a new matter to help prevent or treat a distinctive disease.
The second part is the pre-clinical stage and consists of developing either tissue-culture or cell-culture systems to be tested in animal in order to assess the safety of the candidate vaccine and its immunogenicity (ability to provoke an immune response).
The last part is the IND application and consists of submitting the application for an investigational new drug (IND) which describes the manufacturing and testing process, as well as summarising the laboratory (lab) reports and the proposal study.
Stage 2 of the development is the clinical studies with human subjects. This stage is divided in three phases of vaccine trials where in each phase the size of the sample group increases.
The phase 1 trials consist in the initial human testing involving a small group between 20 and 80 subjects of healthy adults. This trial is considered a non-blinded where all the people involved, including researchers and subjects, all know whether they are given the vaccine or the placebo. The aim of this stage is to access the safety of the candidate vaccine in order to determine the type and extent of the immune response that the vaccine provokes.
The phase 2 trials consist in human testing involving a medium group of 100’s of subjects belonging to the risk group of acquiring the disease. The trial is considered randomised and well controlled which involves the use of a placebo group. The aim of this stage is to study the candidate vaccine in order to determine its safety, immunogenicity, proposed dose, schedule of immunization, and the method of delivery.
Of note, in the case of COVID-19 and due to the stage of pandemic there was an urgency to create the vaccine. Most laboratories reduced the schedules in order to shortened the standard vaccine development time by combining clinical trial steps over months rather than sequentially over years. After phase 2, most countries decided to use the candidate vaccine with an emergency authorization, with the consideration that the phase 3 trials have not been concluded. Every country assumed all the risk involving the vaccine as they were used with incomplete results.
The phase 3 trails consist in human testing involving a large group of 10,000’s of subjects over a wide range of ages, health conditions and ethnic backgrounds. The trial is considered randomised and double-blinded which involves the experimental vaccine being tested against a placebo (saline solution or other substance). The aim of this stage is to assess the vaccine safety, and to detect the rare side effects and the low-frequency events.
Additionally, the vaccine efficiency is tested in order to assess the prevention of the disease, the prevention of infection with pathogens (bacteria, virus or other that causes the disease), the production of antibodies or other type of immune response regarding this pathogen.
Stage 3 of development is the approval and licensure process. This is the time where the vaccine is safe to use as all the studies have been concluded and the results have been published. This stage is divided in three parts consisting in the licensure, post-licensure and further vaccine studies.
The first part is licensure and consists of submitting an approval of the vaccine. This part takes place after a successful phase 3 trial, part of the approval consists of the inspection of the factory where the vaccine will be made and of the labelling of the vaccine.
The second part is post-licensure and consists of the development of a variety of systems in order to monitor the vaccine after they have been approved including the development of phase 4 trails.
The last part is phase 4 trails and consists of developing the optimal studies that drug companies may conduct after the vaccine is released in order to continue evaluating the vaccine for safety, efficacy and other potential uses.
Of note, stage 3 is something that each country has to do for each drug that is going to be use by their citizens. Therefore, this is the reason that some drugs are approved in one country and not in another. This is also the reason that some drugs are used for one disease in one country and for another disease is another country. This process is something that is not just involves science but also politics and economy as one drug made in one country could be cheaper that another.
After this brief research, the topic of vaccines is so complex and has so many implications on top of the science to add the topic of government policy and economic interests. This is why we were never interested about the process of the vaccine before; this was one topic we left to the specialist and we were just applying the vaccine that we were given by our general practitioner (GP).
However, after the chaos caused by this new pandemic and the positive effects that the vaccine has had in the countries that have used them effectively, I thought that a little research of the complexity will be of some use. I also thought, that with a little information about how they are made and the reasons behind each step more people will decided to take the vaccine regardless of the brand as each one has been made to a very high-standard.
In the case of the COVID-19, 15 new vaccines have been developed in the world (as of May 2021). However, it is important to understand that all these vaccines are still undergoing phase 3 trials and most have been approved for emergency use. These new vaccines are classify depending of the type of technology they use which are: mRNA vaccine, adenovirus viral vector vaccine, inactive virus vaccine and protein subunit vaccines.
mRNA-based vaccine (messenger ribonucleic acid) uses a delivery system using a tiny piece of genetic code from SARS CoV-2 to the host cells inside the body which gives instructions to those cells in order to make copies of this spike protein. These proteins stimulate the immune response of the body producing antibodies and developing memory cells which recognise and respond if the body is infected with the actual virus.
Adenovirus viral vector vaccine uses a carrier vaccine made from a modified version of a harmless adenovirus which contains a spike protein found in SARS CoV-2. Once the protein reaches the body’s cell, the immune system starts a defence by creating memory cells in order to protect against the actual SARS CoV-2 infection.
Inactive virus vaccine uses a sample of SARS CoV-2 virus and is grown into large quantities using vero cells (lineage of cells used in cell cultures). These cells are soaked in beta-propiolactone which deactivates the virus by binding to their genes while leaving the other viral particles intact. The resulting inactive viruses are then mixed with an aluminium-based adjuvant (substance that increases the immune response to a vaccine).
Peptide protein subunit vaccine uses three chemically synthesize peptides (short fragments of a viral spike protein) and are merged to a larger carrier protein. This resulting protein is formed by joining a viral nucleocapsid protein and a bacterial MBP (maltose-binding protein) which will target SARS CoV-2 virus in order to trigger the production of protective antibodies.
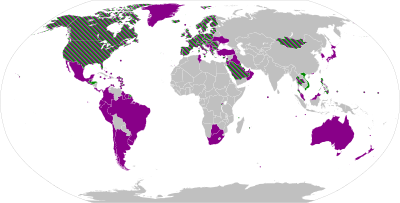
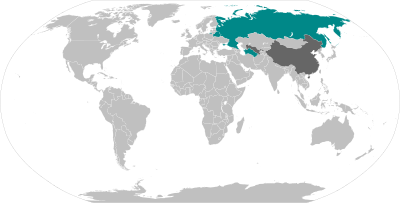
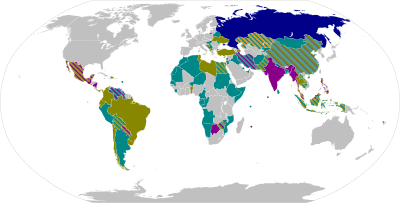
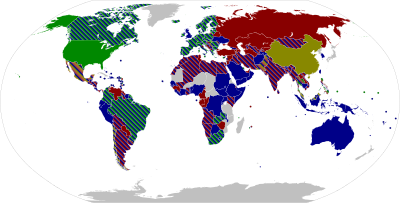
The table below has the list of all the vaccines currently authorised (May 2021) for use against the COVID-19 and are divide into their types and coloured coded according to their manufacturing country.
After analysing the table, it is very easy to choose the one with the highest effectiveness. However, it does not work like that as every country have decided which vaccine should use. Therefore, as a private citizen we do not have a choice of vaccine because the government is the one that buys the doses as the costs per dose are very important.
In comparing the prices per doses from the first 4 vaccines that were developed Oxford-AstraZeneca at £3 ($4) is the cheapest while Moderna at £25 ($33) is the most expensive. In the other two cases, Pfizer-BioNTech is £15($20) and Sputnik V is £7.50 ($10). (source: BBC)
As part of which countries authorised each vaccine, it is important to point out that if the country is involved in the development of the vaccine that country will authorise first the vaccines produced by themselves. This is very clearly depicted by USA (United States of America) which only approved Pfizer-BioNTech, Moderna and Johnson & Johnson. While a country like Mexico which has no local development authorised vaccines from various manufacturing countries like Pfizer-BioNTech, Oxford-AstraZeneca, Sputnik V, CanSino, Sinovac, Covaxin and Johnson & Johnson.
However, one of the main problems of this pandemic was the access to vaccine in a price that every country could get them. It was apparent very early that the richest countries were having earlier access to the vaccines than any other country, therefore, COVAX (COVID-19 vaccines global access) was formed.
COVAX is a worldwide initiative aiming at equitable access to the COVID-19 vaccines directed by the vaccine alliance, the Coalition for Epidemic Preparedness Innovation (CEPI), and the World Health Organisation (WHO) with the goal of coordinate the international resources to enable low-to-middle income countries equitable access to tests, therapies and vaccines all related to COVID-19. By 15th May 2020, there were 165 countries (representing 60% of the world’s population) members of COVAX, however, the shortage of the vaccine has caused serious delays in the vaccination programme of many countries.
This COVID-19 pandemic has had unbelievable statistics worldwide there are 162 million cases with 3.35 million deaths, as of 15th May 2021. Considering that the previous pandemics were also devastating, the table below depicts the latest pandemics the world has suffered.
The influenza is caused by the influenza virus with symptoms including fever, runny nose, sore throat, muscle pain, headache, coughing and fatigue. While the coronavirus is caused by the severe acute respiratory syndrome (SARS) virus with symptoms including fever, dry cough, fatigue, shortness of breath, vomiting, and loss of taste or smell.
I believed that we have been able to contain this pandemic due to the fact that since the Spanish pandemic, the development of the flu vaccines has been a yearly process in order to avoid the major damages and the level of mortality of the Spanish flu. This level of success has been achieved in other pandemics like the Russian flu of 1977 and the Swine flu of 2009, although both deathly, the level of deaths worldwide was reduced significantly.
During this pandemic, this success has also been depicted in the rate of deaths which have been reduced in proportion since the start of the vaccination programme. The highest level of infection occurred after the holiday period of 2020, as many people relaxed all the isolation rules. However, after almost 10 months of vaccine development, the vaccines were ready for deployment in early December 2020.
The first country that started with the vaccination programme was UK and the first vaccine approved for emergency use was the Pfizer-BioNTech. In UK this vaccine was approved on 2nd December 2020 and deployed on 8th December 2020, while in USA was approved on 10th December 2020 and deployed on 14th December 2020. After that date, it was deployed for emergency use by 84 countries including the European Union, Ukraine, Israel, Brazil, Mexico, Japan and Singapore, however, due to the low temperature required for storage many countries have searched for other alternative vaccine.
The Oxford-AstraZeneca was the second vaccine approved worldwide and it was also first approved by UK on 30th December 2020 and deployed on 4th January 2021. After that date, it was then approved by many countries including the European Medicine Agency (EMA), Argentina, Bangladesh, Brazil, Dominican Republic, El Salvador, India, Malaysia, Mexico, Nepal, Pakistan, the Philippines, Sri Lanka and Taiwan, as these vaccines suits hotter countries.
Therefore, UK and USA are the first countries with a heavy vaccination programme and the effects of this mass vaccination are depicted in the dropping of their infection rate.
At the highest peak of infection UK had 68,053 cases on 8th January 2021, by 1st February 2021 the cases dropped 72% to 18,607 cases, by 5th March 2021 the cases dropped a further 71% to 5,455, and by 1st May 2021 the cases dropped a further 65% to 1,907 cases (97.2% lower than the highest peak).
On 15th May 2021, UK has vaccinated with the first dose 53.9% of its population and fully vaccinated 28.3%. Since June 2021, all citizens 25 years old and over are able to get the vaccination if they want, as it is voluntarily.
At the highest peak of infection USA had 208,3009 cases on 10th January 2021, by 2nd February 2021 the cases dropped 67% to 69,744 cases, and by 2nd May 2021 the cases dropped a further 57% to 29,673 cases (85% lower than the highest peak).
On 15th May 2021, USA has vaccinated with the first dose 47.1% of its population and fully vaccinated 36.3%. Since April 2021, all citizens 16 years old and over are able to get the vaccination if they want, as it is voluntarily.
However, countries like Mexico where the vaccination programme has been affected by the irregular supply of vaccination, the infection rate has been impacted but not as heavy as in either UK or USA. At the highest peak of infection Mexico had 20,057 cases on 23rd January 2021, by 6th February 2021 the cases dropped 34% to 13,209 cases, by 1st March 2021 the cases dropped a further 82% to 2,343 cases, and by 2nd May 2021 the cases dropped a further 53% to 1,093 cases (95% lower than the highest peak).
On 15th May 2021, Mexico has vaccinated with the first dose 11.5% of its population and fully vaccinated 7.7%. It is important to note that the huge dropped of cases was caused because the vaccine supply was regulated. Since June 2021, all citizens 40 years old and over are able to get the vaccination if they want, as it is voluntarily.
After everything we have explore about vaccination and all the wonderful benefits that they have in order to reduce the deathliness of an infection disease, these are still drugs which have associated side effects. However, it is important to note that the benefits outweigh the risks but be mindful and vigilant about them.
Therefore, it is important to mention them in order to be vigilant as some are similar to other adult vaccines.
Pfizer-BioNTech common side effects are pain and swelling of the injection site, tiredness, headache, muscle ache, chills, joint paint and fever. However, fever is more common after the second dose.
Oxford-AstraZeneca common side effects like vomiting, diarrhoea, swelling, redness of the injection site, and low levels of blood platelets which occurs in less than 1 in 10 people. The medium side effects are decreased appetite, dizziness, enlarged lymph nodes, sleepiness, sweating, abdominal pain, itching and rash which occurs in less than 1 in 100 people. Finally, the severe side effects are blood clots in combination with low levels of blood platelets which occurs in less 1 in 100,000 people, and anaphylaxis or other allergic reaction.
Moderna common side effects are pain at the injection site, fatigue, headache, muscle pain, and joint pain. The severe side effect is anaphylaxis which occurs in 2.5 cases per million doses and it occurs within 15 minutes of application.
Sputnik V common side effects are flu-like illness, headache, fatigue and injection-site reactions. The severe side effects are deep vein thrombosis, haemorrhagic stroke and hypertension, however, there are no confirmation of those cases were related to the vaccine and there is a huge scepticism due to lack of published raw data or protocol.
Although, the pharma companies have had such a bad reputation for the last 20 years many parents around the world decided that vaccines were more harmful than benefited as they justified this by saying that these companies also do those awful things. However, vaccines are very helpful to children and since they are used worldwide, they are fabricated at a cheap value as otherwise would not sell.
Therefore, these unrelated issues that because they are making a lot of money by selling expensive drugs in order to no use their life-saving vaccine in our kids is nonsense. Vaccinations help reduce the symptoms of very deathly infectious diseases like influenza, COVID-19, pneumonia, osteomyelitis, HIV/AIDS, MMR (measle, mumps, and rubella), smallpox, polio, chickenpox (varicella), tuberculosis (TB), malaria (under development), yellow fever, dengue fever, cholera, and typhoid fever.
Although, some vaccines are only used in countries with high rate of infections for disease like tuberculosis, malaria, yellow fever, dengue fever, and cholera. These vaccines exist but they are difficult to get in some countries so should find a suitable treatment before travelling to those countries. One of these vaccines is dengue fever when I was living in Mexico, I was able to get the dengue fever vaccine and although India has some cases of this disease there is no vaccine available and you do not even get it in UK.
However, the rest of the infectious disease vaccines listed above are applied in every country as part of every person’s vaccination programme. All vaccines have an expiration day but they are all different and they need to be applied at different times of your life.
The idea that only children should get a vaccine is very wrong as most vaccines have an expiration date and adults need a booster for the most serious diseases. However, the influenza vaccine due to their many strains requires a booster every year for everyone specially for people over 60 years old and for younger than 10 years, but if you are able get one every year do it. The vaccination programme established by most governments is as follows:
For kids between birth and 10 years are: MMR (measle, mumps, and rubella), chickenpox, DTaP (diphtheria, tetanus and whooping cough), Hib (influenza type b), polio, PCV (pneumococcal), Hepatitis A & B, RV (rotavirus), and seasonal influenza.
For preteens and teens between 7 and 18 years are: HPV (human papillomavirus), DTaP, MenACWY (meningococcal A/C/W/Y), and seasonal influenza.
For young adults between 19 and 26 years are: seasonal influenza, meningitis, MMR (if born in 1957 or later, 1 or 2 doses), varicella (if born in 1980 or later, 2 doses), PCV (1 dose), pneumococcal polysaccharide (1 or 2 dose), and MenACWY (booster, 1 or 2 doses).
For adults between 27 and 60 years are: seasonal influenza, Td (tetanus and diphtheria with booster every 10 years), HPV (for women between 27 and 45 years old), Tdap (for women each time they are pregnant from 16 weeks), and zoster (after 50 years to prevent shingles, 2 doses).
For older adults from 65 years are: pneumococcal (PPV), seasonal influenza, and Td or Tdap.
The aim of writing this blog is to demonstrate, as a scientist that I am, that vaccines are vital and they need to be applied on time as this schedule is based on the level of infection rate due to their peer group. If I am able to change the mind of one person that vaccines are necessary and voluntarily take the COVID-19 vaccine, I would be very pleased with my actions.
In my case, I had all my early vaccines when I was kid living in Mexico and when travelling to Oaxaca, I got my dengue fever vaccine and booster. When I was planning my first trip to India from UK, I decided to get all the boosters needed to be safe during my trip like hepatitis A, Td (double), polio, and typhoid. I also knew that India has a high rate of infection for malaria so I seek a suitable treatment which was tablet-based. While working with clients face-to-face in the National Health Service (NHS), I needed to keep up with my vaccination programme and I was given vaccines for hepatitis A (1 dose and booster) and for seasonal influenza. Before travelling to Mexico, in October 2020 during this COVID-19 pandemic, I was given the seasonal influenza and the pneumococcal vaccine, as a way of boosting my immune system in order to reduce the coronavirus infection. Since I practice what I preach, once I return to London the first this that I will do will be to book an appointment with my GP after my quarantine period in order to get my COVID-19 vaccine. I am due to have my first GP appointment on 14th June 2021 after the 10 days of mandatory self-isolation due to may stay in an amber colour country, and hopefully while there booked my COVID-19 vaccine.




















Comments